How does it work? Explain the working of acid fire extinguishers with the help of a labeled diagram.
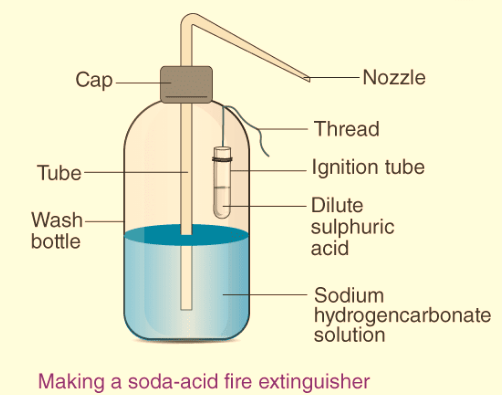
Sulphuric acid and sodium hydrogencarbonate solutions are separated in different containers in a soda fire extinguisher.
The working of the fire extinguisher is described below:
When the fire extinguisher’s knob is pressed once, the sulphuric acid reacts with the sodium hydrogencarbonate solution, releasing carbon dioxide gas. Because of the high pressure in the extinguisher, the carbon dioxide gas will come out as a liquid that falls on the burning object. With the liquid, a blanket of carbon dioxide emerges, cutting off the supply of oxygen to the burning item. The fire goes out when the supply of oxygen is depleted.

