Answer: The correct option is a) return to zero Explanation: When the switch S is closed, the galvanometer's pointer moves to the right. If S is closed, the magnetic field becomes constant, meaning...
The essential difference between an AC generator and a DC generator is that: a) AC generator has an electromagnet while a DC generator has permanent magnet b) DC generator will generate a higher voltage c) AC generator will generate a higher voltage d) AC generator has slip rings while the DC generator has a commutator
Answer: The correct option is d) AC generator has slip rings while the DC generator has a commutator Explanation: So, the main distinction between AC and DC generators is that AC generators have...
The device used for producing electric current is called a: a) generator b) galvanometer c) ammeter d) motor
Answer: The correct option is a) generator Explanation: A Generator is a device that produces an electrical current. Derived from a dynamo. A generator turns mechanical energy into electrical...
The phenomenon of electromagnetic induction is: a) the process of charging a body b) the process of generating magnetic field due to a current passing through a coil c) producing induced current in a coil due to relative motion between a magnet and the coil d) the process of rotating a coil of an electric motor
Answer: The correct option is c) producing induced current in a coil due to relative motion between a magnet and the coil Explanation: According to Faraday's Law of electromagnetic induction, a...
An element X belongs to group 2 and another element Y belongs to group 15 of the periodic table: (a) What is the number of valence electrons in X? (b) What is the valency of X? (c) What the number of valence electrons in Y? (d) What is the valency of Y? Explain how you have arrived at your answers.
(a) 2. For the group 1 and 2, the number of valence electrons is equal to the group number and in the 2nd group, it is equal to 2. (b) 2. Valency is determined by the number of valence electrons...
Nitrogen (atomic number 7) and phosphorus (atomic number 15) belong to group 15 of the periodic table. Write the electronic configurations of these two elements. Which of these will be more electronegative? Why?
The electronic configuration of nitrogen and phosphorous are as follows: N = (2,5) ; P = (2, 8, 5) Nitrogen will have more electronegativity because its atom has small size due to which the...
Fill in the blanks in the following statements: (a) The horizontal rows in a periodic table are called _______. (b) In going across a period (right to left) in periodic table, the atomic size of the atom _______. (c) On moving from right to left in the second period, the number of valence electrons _______. (d) On going down in a group in the periodic table, the metallic character of elements _______. (e) The tendency to gain an electron_______ on moving down in a group of the periodic table.
(a) Periods (b) Increases (c) Decreases (d) Increases (e) Decreases
Rewrite the following statements after correction, if necessary: (i) Elements in the same period have equal valency. (ii) The metallic character of elements in a period increases gradually on moving from left to right.
(i) Elements in the same group have equal valency. (ii)The metallic character of elements in a period decreases gradually on moving from left to right.
A rectangular coil of copper wire is rotated in a magnetic field. The direction of induced current changes once in each: a) two revolutions b) one revolution c) half revolution d) one-fourth revolution
Answer: The correct option is c) half revolution Explanation: As we know that E.M.F is given by: E=nωBAcosωt On every half-rotation, the direction of the induced current changes. As a result, the...
An MCB which cuts off the electricity supply in case of short-circuiting or overloading works on the: a) chemical effect of current b) heating effect of current c) magnetic effect of current d) electroplating effect of current
Answer: The correct option is c) magnetic effect of current. Explanation: A micro circuit breaker (MCB) works faster and more efficiently than a fuse to stop excessive current flow. When heated, an...
Circuit breaker device which can be used in place of fuse in domestic electric wiring is called: a) CBD b) DCB c) MCD d) MCB
Answer: The correct option is d) MCB In place of fuses, MCBs are utilised in home wiring. When the current is high enough, the MCB's electromagnet separates the contacts and breaks the circuit.
A TV set consumes an electric power of 230 watts and runs on 230 volts mains aupply. The correct fuse for this TV set is: a) 5A b) 3A c) 1A d) 2A
Answer: Option (d) 2A is the most adequate answer. Explanation: Power P is equal to 240 watts. V = 240V is the voltage. We already know that power equals voltage times current. Maximum current drawn...
A 3-pin mains plug is fitted to the cable for a 1kW electric kettle to be used on a 250V a.c supply. Which of the following statement is not correct? a) the fuse should be fitted in the live wire b) a 13A fuse is the most appropriate value to use c) the neutral wire is coloured black d) the green wire should be connected to the earth pin
Answer: The correct option is b) a 13A fuse is the most appropriate value to use Explanation: Describing the relationship between the fuse rating and the fuse rating. FR=1.25×PV F R=1.25 \times...
A car headlamp of 48 W works on the car battery of 12V. The correct fuse for the circuit of this car headlamp will be: a) 5A b) 10A c) 3A d) 13A
Answer: The correct option is a) 5A We need a fuse with a current capacity somewhat higher than the device's maximum. According to the stated choice, the correct circuit fuse is 5 A, which is...
Which one of the following statements is not true?
a) In a house circuit, lamps are used in parallel
b) Switches, fuses, and circuit breakers should be placed in the neutral wire
c) An electric iron has its earth wire connected to the metal case to prevent the user receiving a shock
d) When connecting a three-core cable to a 13A three-pin plug, the red wire goes to the live pin
Answer: The correct option is b) switches, fuses, and circuit breakers should be placed in the neutral wire They should not be attached to the neutral wire because if they are, they would all burn...
In normal use, a current of 3.5A flows through a hair dryer. Choose a suitable fuse from the following: a) 3A b) 5A c) 10A d) 30A
Answer: The correct option is b) 5A Explanation: In regular use, a hair dryer draws 3.5 A. 1 to 1.5 are considered safe. So, for 4.2 A, the 5A fuse is safe.
The maximum number of 40W tube-lights connected in parallel which can safely be run from a 240V supply with a 5A fuse is: a) 5 b) 15 c) 20 d) 30
Answer: The correct option is d) 30 Explanation Let n be the number of parallel tube lights that can be safely run. It is given that: Power of one tube-light, $P =40 W$ Therefore, Power of $n$...
A 1.25kW heater works on a 220V mains supply. What current rating would a suitable fuse have? a) 2A b) 5A c) 10A d) 13A
Answer: The correct option is c) 10A Explanation: We need a fuse with a current capacity somewhat higher than the devices. So, 10 A is a little more than the device's current draw of 5.68 A.
At the time of short circuit, the current in the circuit: a) reduces substantially b) does not change c) increases heavily d) varies continuously
Answer: The correct option is c) increases heavily Explanation: Because a short circuit increases the circuit's current, option c is the correct answer. A short circuit is an electrical circuit that...
e) Explain why electric switches should not be operated with wet hands.
Answer: e) It is not recommended to operate electric switches while the hands are wet, due to the fact that water is a good conductor of electricity, and we could receive an electric shock.
c) State the important precautions which should be observed in the use of electricity. d) What will you do if you see a person coming in contact with a live wire?
Answer: c) The following are the most critical safety precautions that should be followed at all times: i) Making use of high-quality cables ii) The use of proper earthing is also important. iii)...
a) Draw a labelled diagram to show the domestic electric wiring from an electric pole to a room. Given the wiring for a bulb and a three-pin socket only. b) State two hazards associated with the use of electricity.
Answer: a) The schematic diagram to show the domestic electric wiring from an electric pole to a room. b) As a result of using electricity, the following two dangers are related to the usage of...
a) Why are fuses fitted in the fuse box of a domestic electricity supply? b) What device could be used in place of the fuses?
Answer: a) For example, fuses installed in a residential electricity supply's fuse box protect the wiring of the house from an excessive current flow in the circuit. b) A fuse is a device that...
a) What current is taken by a 3kW electric geyser working on 240V mains? b) What size fuse should be used in the geyser circuit?
Answer: a) Given, P = 3kW = 3000W V = 240V As we know that, P = VI I = P/V Evaluating the value of current, I = 3000/240 I = 12.5A b) The size of the fuse that should be used in the geyser circuit...
What is the function of an earth wire? Why is it necessary to earth the metallic bodies of electrical appliances?
Answer: To avoid lethal electric shocks, electrical gadgets have iron bodies. When the live wire touches the metallic case, a tremendous quantity of electricity passes through the user's body....
A metal salt MX when exposed to light splits up to form metal M and a gas X2. Metal M is used in making ornaments whereas gas X2 is used in making bleaching powder. The salt MX is itself used in black and white photography.
(a) What do you think metal M is?
(b) What could be gas X2?
(c) Name the metal salt MX.
(d) Name any two salt solutions which on mixing together can produce a precipitate of salt MX.
(e) What type of chemical reaction takes place when salt MX is exposed to light? Write the equation of the reaction.
(a) Silver is the metal X. (a) When MX salt is exposed to light, chlorine is produced as a gas. (c) MX is the metal salt silver chloride that is employed in black and white photography. (d) Silver...
a) An electric iron is rated at 230V, 750W. Calculate i) the maximum current, ii) the number of units of electricity it would use in 30 minutes. b) Which of the following fuse ratings would be suitable for this electric iron? 1A, 3A, 5A, 13A.
Solution: a) Given, V = 230V P = 750W T = 30/60 = 0.5 hours i) Let the maximum current be I As we know that, P = VI Evaluating value of current, 750 = 230I I = 3.26A ii) Electric energy consumed, E...
When a strip of red-brown metal X is placed in a colourless salt solution YNO3 then metal Y is set free and a blue coloured salt solution X(NO3)2 is formed. The liberated metal Y forms a shining white deposit on the strip of metal X.
(a) What do you think metal X is?
(b) Name the salt YNO3.
(c) What could be metal Y?
(d) Name the salt X(NO3)2
(e) What type of reaction takes place between metal X and salt solution YNO3?
(a) Copper is the red-brown strip of metal X. (b) Silver nitrate is a colourless salt solution with the formula YNO3. (c) Silver is the metal that forms a shining white deposit on the strip metal X...
A red-brown metal X forms a salt XSO4. When hydrogen sulphide gas is passed through an aqueous solution of XSO4, then a black precipitate of XS is formed along with sulphuric acid solution.
(a) What could the salt XSO4 be?
(b) What is the colour of salt XSO4?
(c) Name the black precipitate XS
(d) By using the formula of the salt obtained in (a) above, write an equation of the reaction which takes place when hydrogen sulphide gas is passed through its aqueous solution.
(e) What type of chemical reaction takes place in this case?
(a) Copper sulphate is be the salt formed named as XSO4. (b) The salt is blue in colour as it is copper sulphate (c) Copper sulphide is responsible for the black precipitate XS. (d) Chemical...
Two metals X and Y form the salts XSO4 and Y2SO4, respectively. The solution of salt XSO4 is blue in colour whereas that of Y2SO4 is colourless. When barium chloride solution is added to XSO4 solution, then a white precipitate Z is formed along with a salt which turns the solution green. And when barium chloride solution is added to Y2SO4 solution, then the same white precipitate Z is formed along with colourless common salt solution.
(a) What could the metals X and Y be?
(b) Write the name and formula of salt XSO4.
(c) Write the name and formula of salt Y2SO4.
(d) What is the name and formula of white precipitate Z?
(e) Write the name and formula of the salt which turns the solution green in the first case.
(a)Metal X is Copper and Metal Y is Sodium. Both produce sulphate salts on reaction. (b) XSO4 is Copper sulphate, CuSO4 , the salt formed which is blue in colour. (c) Y2SO4 is Sodium sulphate,...
Explain the importance of using in a household electric circuit a) fuse b) earthing wire
Answer: a) As previously said, a fuse is considered to be one of the most significant devices that are used for protection in order to avoid the damages that can occur as a result of either an...
A metal X forms a water soluble salt XNO3. When an aqueous solution of XNO3 is added to common salt solution, then a white precipitate of compound Y is formed alongwith sodium nitrate solution. Metal X is said to be the best conductor of electricity and it does not evolve hydrogen when put in dilute hydrochloric acid.
(a) What is metal X ?
(b) What is salt XNO3?
(c) Name the compound Y.
(d) Write the chemical equation of the reaction which takes place on reacting XNO3 solution and common salt solution giving the physical states of all the reactants and products.
(e) What type of chemical reaction is illustrated by the above equation?
(a) Silver (Ag) is a conductor of electricity that does not produce hydrogen when exposed to dilute hydrochloric acid. (b) Silver nitrate (AgNO3) is XNO3 which reacts with NaCl (common salt) to give...
When a black metal compound XO is heated with a colourless gas Y2, then metal X and another compound Y2O are formed. Metal X is red-brown in colour which does not react with dilute acids at all. Gas Y2 can be prepared by the action of a dilute acid on any active metal. The compound Y2O is a liquid at room temperature which can turn anhydrous copper sulphate blue.
(a) What do you think is metal X?
(b) What could be gas Y 2?
(c) What is compound XO?
(d) What is compound Y2O?
(e) Write the chemical equation of the reaction which takes place on heating XO with Y2.
(f) What type of chemical reaction is illustrated in the above equation?
(a) Metal X is Copper (Cu) which is red-brown in colour and does not react with dilute acids. (b) Colorless gas Y2 is Hydrogen (H2). (c) Compound XO, a black metal compound is a Copper oxide (CuO)...
a) When does a fuse cut off current? How does it do it? b) What is the maximum number of 60W bulbs that can be run from the mains supply of 220 volts if you do not want to overload a 5A fuse?
Answer: a) When the current exceeds the safe limits, the fuse wire melts and breaks the circuit. This is called a fuse turned off. b) Given, V = 220V I = 5A Let the maximum number of bulbs be y...
A strip of metal X is dipped in a blue coloured salt solution YSO4. After some time, a layer of metal Y from the salt solution is formed on the surface of metal strip X. Metal X is used in galvanisation whereas metal Y is used in making electric wires. Metal X and metal Y together form an alloy Z.
(a) What could metal X be?
(b) What could metal Y be?
(c) Name the metal salt YSO4
(d) What type of chemical reaction takes place when metal X reacts with salt solution YSO4? Write the equation of the chemical reaction involved.
(e) Name the alloy Z.
(a) Zn undergoes displacement reaction and is also used for galvanization. The evidences suggest that metal X is Zinc (Zn). (b) Copper is more reactive than Zn and displaces it while interacting....
Distinguish between the terms ‘overloading’ and ‘short-circuiting’ as used in domestic circuits.
Answer: A short circuit occurs when the live wire comes into touch with the neutral wire, which is a dangerous situation. It is known as overloading when more than two electrical appliances with a...
When hydrogen burns in oxygen, water is formed and when water is electrolysed, then hydrogen and oxygen are produced. What type of reaction takes place?
(a) In the first case?
(b) In the second case?
(a) The formation of water as a result of a reaction involving hydrogen burning in oxygen gas is a type of combination reaction. (b) The decomposition reaction takes place in the second case...
What type of electric fuse is used in electrical appliances like car stereos? Explain with the help of a labelled diagram.
Answer: Cartridge fuses are used in automobile stereos. An inner glass tube T contains a tiny fuse wire. There are metal caps on the glass tube. The metal cap connects the two fuse ends. These metal...
A colourless lead salt, when heated, produces a yellow residue and brown fumes.
(a) Name the lead salt.
(b) Name the brown fumes.
(c) Write a chemical equation of the reaction involved
(a) Lead salt here is Lead nitrate. (b) Brown fumes are produced due to the production of Nitrogen dioxide. (c) Chemical equation for the reaction involved is: 2Pb(NO3)2→ 2PbO + 4NO2
When a green iron salt is heated strongly, its colour finally changes to brown and odour of burning sulphur is given out.
(a) Name the iron salt.
(b) Name the type of reaction that takes place during the heating of iron salt.
(c) Write a chemical equation for the reaction involved.
(a) Ferrous sulphate. (b) Decomposition reaction. Anhydrous ferrous sulphate is formed when a ferrous sulphate crystal loses water. It decomposes into ferric oxide, sulphur dioxide, and sulphur...
a) Of what substance is the fuse wire made? Why? b) Explain why a copper wire cannot be used as a fuse wire?
Answer: a) Fuse wire is made of tin-plated copper, which has a low melting point. b) Pure copper has a high melting point, making it harder to melt during short circuits.
You are given the following chemical equation:
![Rendered by QuickLaTeX.com \[\mathbf{Mg}\left( \mathbf{s} \right)\text{ }+\text{ }\mathbf{CuO}\left( \mathbf{s} \right)~\to \mathbf{MgO}\left( \mathbf{s} \right)\text{ }+\text{ }\mathbf{Cu}\text{ }\left( \mathbf{s} \right)\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-6f995beaa02cb60c6c6f41fd202b467a_l3.png)
(a) Decomposition reaction as well as displacement reaction
(b) Combination reaction as well as double displacement reaction
(c) Redox reaction as well as displacement reaction
(d) Double displacement reaction as well as Redox reaction
The answer is option(c). Because magnesium is oxidised here and copper is reduced, a redox reaction. Also, Magnesium being more reactive displaces copper in the solution. As a result, displacement...
Consider the reaction:
![Rendered by QuickLaTeX.com \[\mathbf{KBr}\text{ }\left( \mathbf{aq} \right)\text{ }+\text{ }\mathbf{AgN}{{\mathbf{O}}_{\mathbf{3}~}}\left( \mathbf{aq} \right)\text{ }\to \text{ }\mathbf{KN}{{\mathbf{O}}_{\mathbf{3}~}}\left( \mathbf{aq} \right)\text{ }+\text{ }\mathbf{AgBr}\text{ }\left( \mathbf{s} \right)\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-1426359ca96caabcf611532f6230d500_l3.png)
This is an example of:
(a) Decomposition reaction
(b) Combination reaction
(c) Double displacement reaction
(d) Displacement reaction
Option (c) is the correct response. The given reaction is a type of double displacement reaction, in which ions are exchanged between both the reactants.
Which of the following can be decomposed by the action of light?
(a) NaCl
(b) KCl
(c)AgCl
(d) CuCl
Option (c) is the correct answer. A decomposition reaction is a reaction in which reactants break down into simpler products. Here, silver chloride is the substance that can be decomposed by the...
The process of respiration is:
(a) An oxidation reaction which is endothermic
(b)A reduction reaction which is exothermic
(c) A combination reaction which is endothermic
(d) An oxidation reaction which is exothermic
Option (d) is the correct response. The food we eat is digested and converted into energy during digestion. Carbohydrate-rich foods are broken down into glucose, which then combines with oxygen to...
Consider the following equation of the chemical reaction of a metal M:
![Rendered by QuickLaTeX.com \[\mathbf{4M}\text{ }+\text{ }\mathbf{3}{{\mathbf{O}}_{\mathbf{2}}}\to \text{ }\mathbf{2}{{\mathbf{M}}_{\mathbf{2}}}{{\mathbf{O}}_{\mathbf{3}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-b55903fe466d8c12a229da84a4a44476_l3.png)
The equation represents:
(a) Combination reaction as well as reduction reaction
(b) Decomposition reaction as well as oxidation reaction
(c) Oxidation reaction as well as displacement reaction
(d) Combination reaction as well as oxidation reaction
Option (d) is the correct response. Metal combines with oxygen to produce a single compound in the above reaction. This is a type of combination reaction, and since oxygen is added, thus it is an...
A white precipitate will be obtained if we add common salt solution to:
![Rendered by QuickLaTeX.com \[\left( \mathbf{a} \right)\text{ }\mathbf{Ba}{{(\mathbf{N}{{\mathbf{O}}_{\mathbf{3}}})}_{\mathbf{2}}}\mathbf{solution}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-50da9bbc9460443f2e88d01ecb83dbc3_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{b} \right)\text{ }\mathbf{KN}{{\mathbf{O}}_{\mathbf{3}}}~\mathbf{solution}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-b0c35f69d350ff362bf0b0da57705207_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{c} \right)\mathbf{AgN}{{\mathbf{O}}_{\mathbf{3}}}\mathbf{solution}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-a1419bea63bda4ead8c43a88602a7240_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{d} \right)\text{ }\mathbf{Mg}{{(\mathbf{N}{{\mathbf{O}}_{\mathbf{3}}})}_{\mathbf{2}}}~\mathbf{solution}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-08e89344a0a4ccac9e6363e81f42a6d7_l3.png)
Option (c) is the correct response. When we add common salt to silver nitrate, a precipitation reaction occurs to give a white precipitate.
A white precipitate can be obtained by adding sulphuric acid to:
(a) CuSO4 solution
(b) NaCl solution
(c)BaCl2 solution
(d) Na2SO4 solution
Option (c) is the correct answer. When dilute sulphuric acid reacts with barium chloride, barium sulfate is produced along with HCl gas. The resulting barium sulfate is a white, insoluble precipitate.
In order to prevent the spoilage of potato chips, they are packed in plastic bags in an atmosphere of:
(a) Cl2
(b)H2
(c)N2
(d) O2
Option (c) is the correct response. Food items packed in plastic bags in a nitrogen gas atmosphere do not get spoiled since nitrogen is an inert gas that does not react with the items. This is done...
The chemical reaction involved in the corrosion of iron metal is that of:
(a) Oxidation as well as displacement
(b) Reduction as well as combination
(c) Oxidation as well as combination
(d) Reduction as well as displacement
Option (c) is the correct response. Corrosion occurs when iron metal combines with water and oxygen to generate hydrated oxide. This is a combination reaction and also an oxidation reaction since...
In the context of redox reactions, the removal of hydrogen from a substance is known as :
(a) Oxidation
(b) Dehydration
(c) Reduction
(d) Dehydrogenation
Option (a) is the correct response. Redox reactions involve both reduction and oxidation, with reduction involving the addition of hydrogen and oxidation involving the removal of hydrogen.
The removal of oxygen from a substance is called
(a) Oxidation
(b) Corrosion
(c)Reduction
(d) Rancidity
Option (c) is the right answer. The process of removing oxygen from a substance and replacing it with hydrogen is known as reduction. It occurs when the rate of atom oxidation drops.
(a) What happens when an aqueous solution of sodium sulphate reacts with an aqueous solution of barium chloride?
(b) Write the balanced chemical equation for the reaction which takes place.
(c) State the physical conditions of reactants in which the reaction will not take place.
(d) Name the type of chemical reaction which occurs.
(e) Give one example of another reaction which is of the same type as the above reaction.
(a) When barium chloride solution is mixed with sodium sulphate solution, a white barium sulphate precipitate forms along with the sodium chloride solution. Na2SO4 + BaCl2 → BaSO4 + 2NaCl (c) Solid...
Fill in the following blanks with suitable words: a) A fuse should always be placed in the …….. wire of a mains circuit. b) The earth wire should be connected to the ….. of an appliance.
Answer: a) Live b) Body. Explanation: a) If the fuse is linked to a neutral wire rather than a live wire, and the fuse blows, the appliances remain connected to the live wire and the current...
How should the electric lamps in a building be connected so that the switching on or off in a room has no effect on other lamps in the same building?
Answer: The parallel connection should be used to connect all of the bulbs in a structure.
Give two reasons why different electrical appliances in a domestic circuit are connected in parallel.
Answer: Two reasons exist for connecting electrical appliances in parallel in a household circuit: a) When connected in parallel, when one stops working, the others continue to work. b) All...
What is the main purpose of earthing an electrical appliance?
Answer: Generally speaking, the primary goal of earthing electrical equipment is to reduce the possibility of electric shocks.
What is the usual colour of the insulation of a) live wire b) neutral wire c) earth wire?
Answer: a) Red is the colour of live wire b) Black wire is used for the neutral wire. c) The green earth wire serves as the grounding line.
Usually three insulated wires of different colours are used in an electrical appliances. Name the three colours.
Answer: Following are the three colours that are used in an electric appliances: a) Red b) Black c) Green
In household circuit, is a fuse wire connected in series or in parallel?
Answer: To safeguard the circuit from overcurrent, fuses are always connected in series. A blown fuse will open the circuit and stop the flow of current.
In which wire in an AC housing circuit is the switch introduced to operate the lights?
Answer: Live wire Switches are installed in the live wire to allow an electrical device to be turned off and on. Switches are used to connect an electrical device to the main power supply. As long...
When does an electric short circuit occur?
Answer: In order for a short circuit to occur, the live wire and the neutral wire must come into direct contact with one another.
If fuses of 250mA, 500mA, 1A, 5A, and 10A were available, which one would be the most suitable for protecting an amplifier rated at 240V, 180W?
Solution: Given, P = 180W V = 240V As we know that, P = VI Thus, evaluating the value of I I = P/V = 180/240 = 0.75 A Because of this, the fuse wire should be capable of withstanding currents up to...
What is the electric potential of the neutral wire in a mains supply cable?
Answer: The electric potential of the neutral wire in a mains supply cable is zero volts. In an AC circuit, the neutral wire serves as a return path for the passage of electricity. The neutral wire...
List the colours of the three wires in the cable connected to the plug of an electric iron.
Answer: The three wires of the cable that connects to the plug of an electric iron are colored as follows: red, black, and blue. a) Red wire: This is a live wire. b) The black wire is the neutral...
Along with live wire and neutral wire, a third wire is also used in domestic electric wiring, what name is given to this third wire?
Answer: The earth wire, which is used in conjunction with the live wire and neutral wire, is the third wire that is employed.
State whether the following statements are true or false: a) A wire with a green insulation is usually the live wire b) A miniature circuit breaker (MCB) works on the heating effect of current
Answer: a) False Explanation The electric power line enters our house via three wires: live, neutral, and earth. The first two transport electricity from the power plant, while the third is earthed...
Give the symbol of an electric fuse used in circuit diagram.
The circuit diagram uses the following symbol for an electric fuse:
What is the usual capacity of an electric fuse used in the a) lighting circuit and b) in the power circuit of a small house?
Answer: Following is the usual capacity of an electric fuse that is used in: a) 5A b) 15A It is a stated fact.
What name is given to the device which automatically cuts off the electricity supply during short-circuiting in household wiring?
Answer: The term "electric fuse" refers to the device that is used to automatically switch off the electricity supply when a short circuit occurs in the domestic wiring.
a) What do you understand by the terms ‘direct current’ and ‘alternating current’? b) Name some sources of direct current and some of alternating current. c) State an important advantage of alternating current over direct current. d) What is the frequency of AC supply in India?
Answer: a) As a general rule, direct current is used to refer to the current that flows in just one direction, and alternating current is used to refer to the current that flows in both directions...
a) What do you understand by the term ‘electromagnetic induction’? Explain with the help of a diagram. b) Name one device which works on the phenomenon of electromagnetic induction. c) Describe different ways to induce current in a coil of wire.
Answer: a)Electricity is produced by electromagnetism, which is referred to as electromagnetic induction. The wire AB should be moved upwards between the poles of the horseshoe magnet so that it is...
Draw the labelled diagram of an AC generator. With the help of this diagram, explain the construction and working of an AC generator.
Answer: Construction: A rectangular coil ABCD is rapidly rotated between the north and south poles of a powerful horseshoe magnet. A permanent magnet M is utilised. The coil with several twists is...
a) In what respect does the construction of an AC generator differ from that of a DC generator? b) What normally drives the alternators in a thermal power station? What fuels can be used to heat water in the boiler?
a) When it comes to the building of both the DC and the AC generators, the ends of both generator coils are connected to the outer circuit. A DC generator is comprised of two half rings of copper...
Name and state the rule to find the direction of: a) current induced in a coil due to its rotation in a magnetic field b) force experienced by a current-carrying straight conductor placed in a magnetic field which is perpendicular to it.
Answer: a) Fleming's right-hand rule: The thumb, forefinger, and middle finger of the right hand are parallel. The thumb indicates conductor velocity, the forefinger the magnetic field, and the...
State and explain Fleming’s right hand rule.
Answer: The direction of induced current in a magnetic field is given by Fleming's right-hand rule. The right thumb, forefinger, and center finger must be held at right angles. The forefinger shows...
a) What is the difference between alternating current and direct current? b) What type of current is given by i) a dry cell ii) a power house generator?
Answer: a) i) The distinction between alternating current and direct current is that alternating current can flow in both directions. ii) DC current's value and direction remain constant while AC...
a) Explain the principle of an electric generator. b) State two ways in which the current induced in the coil of a generator could be increased.
Answer: a) An electric generator works by moving a straight conductor in a magnetic field, causing current to flow. b) Current is induced in a generator's coil in the following ways: i) Faster coil...
Two circular coils A and B are placed closed to each other, if the current in the coil A is changed, will some current be induced in the coil B? Give reason for your answer.
Answer: Because of the shift in the magnetic field caused by the placement of coils B and C in close proximity to each other, the other coil will experience some current induced into it....
Complete the following sentence: A generator with commutator produces …… current.
Answer: A generator with a commutator produces a direct current.
List three sources of magnetic fields.
Answer: The three primary sources of magnetic field generation are as follows: Permanent magnet Electromagnets A conductor that transports current.
Why are thermal power stations usually located near a river?
Answer: Heat-generating plants, such as thermal power plants, are typically placed near rivers because they require a large and consistent supply of water in order for water to be transformed into...
What change should be made in an AC generator so that it may become a DC generator?
Answer: To convert an alternating current generator into a direct current generator, the slip rings must be replaced with a commutator.
What type of generator is used at power stations?
Answer: An unstable potential difference is produced by an alternating current generator. E.g. A coil of wire moving in a magnetic field is an ac generator. At power plants, alternating current...
What condition is necessary for the production of current by electromagnetic induction?
Answer: The movement of the wire in relation to the magnet is a necessary condition for the generation of current through electromagnetic induction to occur.
In the Modern Periodic Table, calcium (atomic number 20) is surrounded by elements with atomic numbers 12, 19, 21 and 38. Which of these have physical and chemical properties resembling calcium?
In the Modern Periodic Table, the element calcium has an atomic number 20, so it has 2, 8, 8, 2 electronic configurations. Therefore, this two valance electron containing element is identical to the...
How does the electronic configuration of an atom relate to its position in the Modern Periodic Table?
In the Modern Periodic Table, the electronic configuration of an element determines the number of valence electrons whereas the position of the atom is determined by the valence electrons.
Nitrogen (atomic number 7) and phosphorus (atomic number 15) belong to group 15 of the Periodic Table. Write the electronic configuration of these two elements. Which of these will be more electronegative? Why?
Nitrogen: Atomic number - 7 Electronic configuration - 1s2 2s2 2p3 Nitrogen: Atomic number - 15 Electronic configuration - 1s2 2s2 2p6 3s2 3p3 The number of shell increases in the periodic table...
The position of three elements A, B and C in the Periodic Table are shown below–
Group 16 Group 17 - - - A - - B C (a) State whether A is a metal or non-metal. (b) State whether C is more reactive or less reactive than A. (c) Will C be larger or smaller in size than B?...
An atom has electronic configuration 2, 8, 7. (a) What is the atomic number of this element? (b) To which of the following elements would it be chemically similar? (Atomic numbers are given in parentheses.) N(7), F(9), P(15), Ar(18)
(a)The element is Chlorine (Cl) and the atomic number of chlorine is 17. (b) The atom with the electronic configuration of 2, 8, 7 which is the chlorine is chemically identical to Fluorine (F) which...
(a) What property do all elements in the same column of the Periodic Table as boron have in common? (b) What property do all elements in the same column of the Periodic Table as fluorine have in common?
(a) All the elements are in the same boron column (group 13). Thus, they have three electrons in their valence shells. Besides, non-metallic boron, all other elements (i.e., aluminum, gallium,...
Which element has? (a) Two shells, both of which are completely filled with electrons? (b) The electronic configuration 2, 8, 2? (c) A total of three shells, with four electrons in its valence shell? (d) A total of two shells, with three electrons in its valence shell?(e) twice as many electrons in its second shell as in its first shell?
(a) Neon (Ne) has two shells in which both are completely filled with electrons. (b) Magnesium (Mg) has the electronic configuration 2, 8, 2. (c) Silicon (Si) has a total of three shells, with four...
Element X forms a chloride with the formula XCl2, which is a solid with a high melting point. X would most likely be in the same group of the Periodic Table as (a) Na (b) Mg (c) AI (d) Si
Identification: Element X - Magnesium (Mg) containing valency of 2. Explanation: When Mg is combined with chloride (Cl), it forms Magnesium Chloride (MgCl2).
Which of the following statements is not a correct statement about the trends when going from left to right across the periods of Periodic Table. (a) The elements become less metallic in nature. (b) The number of valence electrons increases. (c) The atoms lose their electrons more easily. (d) The oxides become more acidic
Correct Answer: (c) The atoms lose their electrons more easily Explanation: Atoms do not lose their electrons easily because as we move from left to right across the periodic table, the non-metallic...
By considering their position in the Periodic Table, which one of the following elements would you expect to have maximum metallic characteristic? Ga/Ge/As/Se/Be
The elements that are expected to have the most metallic character are Be and Ga. The tendency to lose electrons is greater in Ga than the Be as the size of Ga is bigger. Thus, the element Ga have...
In the Modern Periodic Table, which are the metals among the first ten elements?
The metals among the first ten elements in the modern periodic table are, Lithium Beryllium
(a) Lithium, sodium, potassium are all metals that react with water to liberate hydrogen gas. Is there any similarity in the atoms of these elements? (b) Helium is an unreactive gas and neon is a gas of extremely low reactivity. What, if anything, do their atoms have in common?
(a) Lithium, sodium, potassium are the metals that are unstable because of the unfilled outermost shells. This is the reason that they react with water in order to release hydrogen and these metals...
Name 1. Three elements that have a single electron in their outermost shells. 2. Two elements that have two electrons in their outermost shells. 3. Three elements with filled outermost shells
Lithium (Li), Sodium (Na) and potassium (k) are the three elements that have only one electron in their outermost shells. Magnesium (Mg) and Calcium (Ca) are the two elements that contains...
Name two elements you would expect to show chemical reactions similar to magnesium. What is the basis for your choice?
I think that the elements Calcium and Beryllium are expected to show chemical reactions that are similar to the element magnesium as these elements belong to the same group having 2 valence...
How could the Modern Periodic Table remove various anomalies of Mendeleev’s Periodic Table?
The Modern periodic table is arranged in order of their atomic number. This removes inconsistencies in relation to certain pairs of the elements present in the Mendeleev’s periodic table. The atomic...
Why do you think the noble gases are placed in a separate group?
The noble gases have been classified as separate group because of their inert nature as they are also called as inert gases. Noble gases are present in low concentration in our atmosphere. They are...
What were the criteria used by Mendeleev in creating his Periodic Table?
Mendeleev focused on a variety of chemicals made up of elements containing Hydrogen and Oxygen. Among the material, he saw the interrelationships between the mass of many atoms of various elements...
Besides gallium, which other elements have since been discovered that were left by Mendeleev in his Periodic Table? (Any two)
The elements that are left by the Mendeleev’s Periodic Table besides gallium are, Germanium Scandium
Use Mendeleev’s Periodic Table to predict the formulae for the oxides of the following elements: K, C, AI, Si, Ba.
According to the Mendeleev’s Periodic Table, Potassium (K) belongs to group IA with a valency of 1. Formula - K2O Carbon (C) belongs to group IV A with a valency of 4. Formula - C2O4 or CO2 Aluminum...
What were the limitations of Newlands’ Law of Octaves?
Limitations of Newlands’ Law of Octaves: Newlands Octave law applies only to the elements till Calcium. Newland estimates that there are a total of 56 elements in nature and that there are no more...
Compare and contrast the arrangement of elements in Mendeleev’s Periodic Table and the Modern Periodic Table.
Mendeleev’s Periodic Table Modern Periodic Table In the Mendeleev’s Periodic Table, the arrangement of the elements are according to the increasing order of the element’s atomic mass. In the Modern...
What were the limitations of Döbereiner’s classification?
Limitations of Döbereiner’s classification: They do not work at elements which have very low or high mass. All elements could not fit into Dobereiner's traids. As the methods of calculating the...
Explain the mechanism of the cleaning action of soaps.
There is a lot of dirt and contaminants mixed with water, and most of all the impurities do not dissolve in the water. Soap molecules are a mixture of salts such as sodium or potassium. Molecules...
Give a test that can be used to differentiate between saturated and unsaturated hydrocarbons.
The Bromine Water Testing is used to distinguish between unsaturated chemicals such as alkenes and alkynes and saturated chemicals. For this purpose, bromine is used in the form of water. The...
Which of the following hydrocarbons undergo addition reactions: C2H6, C3H8, C3H6, C2H2 and CH4.
The hydrocarbons that undergo addition reactions are: C3H6 C2H2
What is hydrogenation? What is its industrial application?
Hydrogenation is the process or chemical reaction between hydrogen and other chemicals. It is usually done in the presence of catalysts like for example nickel, palladium or platinum. Hydrogenation...
What change will you observe if you test soap with litmus paper (red and blue)?
When soap is dissolved in water, due to the formation of alkaline NaOH or KOH, the solution is also alkaline. The solution turns red litmus into blue, but in soap solution, blue litmus do not change...
Explain the formation of scum when hard water is treated with soap?
A scrum is produced by a reaction of hard water with soap. The calcium and magnesium present in hard water form an insoluble white precipitate (scrum).
Why are carbon and its compounds used as fuels for most applications?
Carbon and its compounds used as fuel for many applications because they have high calorie values and provide a lot of energy. Most carbon dioxide emits a lot of heat and light when burned in the...
Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also?
Micelle formation occurs due to contaminated particles in water and fresh water. There are two departments involved: one is pure water and the other is impurities. Soap also has two modes (organic...
How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties?
Ethanol Ethanoic acid Ethanol do not react with sodium hydrogen carbonate Ethanoic acid reacts with sodium hydrogen carbonate Ethanol has a good smell and a burning taste Ethanoic acid has a vinegar...
What is a homologous series? Explain with an example.
A homologous series is a series of chemical compounds having same functional group, general formula and chemical properties. As there is a change in physical structures, we can say that there will...
Draw the electron dot structures for (a) ethanoic acid (b) H2 S (c) propanone (d) F2
(a) Electron dot structure of ethanoic acid: (b) Electron dot structure of H2S: (c) Electron dot structure of propanone: (d) Electron dot structure of F2:
Explain the nature of the covalent bond using the bond formation in CH3Cl
Carbon cannot lose four electrons or gain four electrons as these processes make the system unstable due to the need for additional energy. CH3Cl therefore eliminates its octet suspension by sharing...
While cooking, if the bottom of the vessel is getting blackened on the outside, it means that (a) the food is not cooked completely. (b) the fuel is not burning completely. (c) the fuel is wet. (d) the fuel is burning completely.
Correct Answer: (b) the fuel is not burning completely Explanation: While cooking, if the bottom of the vessel is dark outside it shows that the fuel is not completely burning.
Butanone is a four-carbon compound with the functional group (a) carboxylic acid (b) aldehyde (c) ketone (d) alcohol
Correct Answer: (c) ketone Explanation: Butanone, also known as methyl ethyl ketone, is an organic chemical compound manufactured on a large scale, but only happens in the environment by tracing.
Ethane, with the molecular formula C2H6 has (a) 6 covalent bonds. (b) 7 covalent bonds. (c) 8 covalent bonds. (d) 9 covalent bonds
Correct Answer: (b) 7 covalent bonds Explanation: Ethane is the simplest hydrocarbon containing a single carbon-carbon bond. The second most important source of natural gas, it is also dispersed in...
People use a variety of methods to wash clothes. Usually after adding the soap, they ‘beat’ the clothes on a stone, or beat it with a paddle, scrub with a brush or the mixture is agitated in a washing machine. Why is agitation necessary to get clean clothes?
Usually, agitation is necessary to obtain clean clothes such as agitation aid soap micelles to hold oil, grease or any other impurities to be removed. When the clothes are agitated or beaten, the...
Would you be able to check if water is hard by using a detergent?
It is impossible to check whether the water is hard by using a detergent because the detergents are made of ammonium salts or long carboxylic acid sulphonates. Unlike soaps do not work with calcium...
What are oxidising agents?
Oxidising agents are compounds that remove Hydrogen or add oxygen to a mixture. Eg: halogen, potassium nitrate, and nitric acid.
How would you distinguish experimentally between an alcohol and a carboxylic acid?
In reaction with Sodium Carbonate, Carboxylic acid produces carbon dioxide gas which converts lime water into a milky form and alcohol does not give this reaction. This test can be used to separate...
A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
The mixture of oxygen and ethyne is heated instead of a mixture of ethyne and air because heat production is very important in heating metals. When oxygen and ethyne burn, they completely burn to...
Why is the conversion of ethanol to ethanoic acid an oxidation reaction?
The conversion of ethanol to ethanoic acid involves the removal of the Hydrogen atom and the addition of oxygen (oxidation reaction). In the first step, the Hydrogen molecule is removed from ethanol...
How would you name the following compounds?
1. CH3—CH2—Br 2. 3. Answers: 1. Bromoethane 2. Methanal or Formaldehyde 3. 1 – Hexyne
Draw the structures for the following compounds. (i) Ethanoic acid (ii) Bromopentane (iii) Butanone (iv) Hexanal
(i) Ethanoic acid: (ii) Bromopentane: (iii) Butanone: (iv) Hexanal:
What will be the formula and electron dot structure of cyclopentane?
Formula and Electron dot structure of cyclopentane:
What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
Carbon has six electrons in valence which is actually the highest number of valency. The Covalent bond occurs easily with carbon atoms and many others such as oxygen, chlorine, nitrogen, sulfur,...
How many structural isomers can you draw for pentane?
1. n-pentane: 2. 2-methylbutane: 3. 2, 2-dimethylpropane:
What would be the electron dot structure of a molecule of Sulphur which is made up of eight atoms of Sulphur? (Hint – The eight atoms of Sulphur are joined together in the form of a ring).
Electron dot structure of a molecule of Sulphur:
What would be the electron dot structure of carbon dioxide which has the formula CO2?
Electron dot structure of carbon dioxide (CO2):
(a) Explain the term “rancidity”. What damage is caused by rancidity?
(b) What type of chemical reaction is responsible for causing rancidity?
(c) State and explain the various methods for preventing or retarding rancidity of food.
(a)Rancidity is the result of the slow oxidation of oil and fat found in food products, which produces a bad odour and taste. When fats and oils oxidise, the food turns rancid, which affects the...
(a) Explain the term “corrosion” with an example. Write a chemical equation to show the process of corrosion of iron.
(b) What special name is given to the corrosion of iron?
(c) What type of chemical reaction is involved in the corrosion of iron?
(d) Name any three objects (or structures) which are gradually damaged by the corrosion of iron and steel.
Corrosion is a natural process that gradually degrades things in the environment through chemical or electrochemical reactions. Corrosion happens when the majority of all of the atoms on a metal...
What happens when a zinc strip is dipped into a copper sulphate solution?
(a) Write the equation for the reaction that takes place.
(b) Name the type of reaction involved.
When a strip of zinc metal is immersed in copper sulphate solution, zinc sulphate solution and copper are obtained. (a) The chemical equation for the reaction mentioned above is as follows:-...
What happens when silver chloride is exposed to sunlight? Write a chemical equation for this reaction. Also give one use of such a reaction.
When silver chloride is exposed to light, it decomposes into silver metal and chlorine gas, which is a photolytic breakdown reaction. The chemical equation for this reaction is as follows:- 2AgCl(s)...
What happens when silver nitrate solution is added to sodium chloride solution?
(a) Write the equation for the reaction which takes place.
(b) Name the type of reaction involved.
When silver nitrate solution is added to sodium chloride solution, a white silver chloride precipitate forms along with the sodium nitrate solution. (a) The chemical equation for the reaction is:-...
(a) What happens when a piece of iron metal is placed in copper sulphate solution? Name the type of reaction involved.
(b) Write balanced chemical equation with state symbols for the following reaction:
Barium chloride solution reacts with sodium sulphate solution to give insoluble barium sulphate and a solution of sodium chloride.
(a) Magnesium sulphate solution and copper metal are produced when a piece of iron metal is immersed in a copper sulphate solution. This is an example of a displacement reaction. CuSO4 + Fe →...
(a) Why are decomposition reactions called the opposite of combination reactions? Explain with equations of these reactions.
(b) Express the following facts in the form of a balanced chemical equation:
“When a strip of copper metal is placed in a solution of silver nitrate, metallic silver is precipitated and a solution containing copper nitrate is formed.”
(a) Decomposition reactions are reactions that split or break substances into two simpler components. When calcium carbonate is heated, it decomposes into calcium oxide and carbon dioxide. This is...
What is meant by (a) displacement reaction, and (b) double displacement reaction? Explain with the help of one example each.
(a) A displacement reaction occurs when one active element removes a less reactive ingredient from a salt solution. 2KI + Cl2 → 2KCl + I2 b) A double displacement reaction occurs when two ionic...
(a) Define the following in terms of gain or loss of hydrogen with one example each:
(i) Oxidation (ii) reduction
(b) When a magnesium ribbon is heated, it burns in air to form magnesium oxide. Write a balanced chemical equation for this reaction. Name (i) substance oxidised, and (ii) substance reduced.
Oxidation is the removal of hydrogen and reduction is the addition of hydrogen. This is demonstrated by an example as foloows: Fe2O3 + 3CO → 2Fe + 3CO2 In the reaction above, Fe got reduced while...
(a) Explain the following in terms of gain or loss of Oxygen with one example each:
(i) Oxidation (ii) reduction
(b) When copper powder is heated strongly in air, it forms copper oxide. Write a balanced chemical equation for this reaction.
Name
(i) substance oxidised, and
(ii) substance reduced
(a) Oxidation is the addition of oxygen while reduction is the removal of oxygen. H2S + Cl2→ S + 2HCl In the reaction shown above, hydrogen is removed from hydrogen sulphide, so it is an oxidation...
(a) What is the difference between displacement and double displacement reactions? Write equations for these reactions.
(b) What do you mean by a precipitation reaction? Explain giving an example.
(a) A displacement reaction occurs when a more reactive ingredient displaces a less reactive element out of its compound. The displacement reaction involves both metals and non-metals. An example of...
Give one example of an oxidation-reduction reaction which is also:
(a) A combination reaction
(b) A displacement reaction
(a) 2Cu + O2 → 2CuO (in the presence of heat) Copper oxide is formed when copper reacts with oxygen in an oxidation-reduction reaction. (b) CuO + H2→ Cu + H2O Dihydrogen oxide is formed when...
Zinc oxide reacts with carbon, on heating, to form zinc metal and carbon monoxide. Write a balanced chemical equation for this reaction. Name
(i) oxidizing agent, and
(ii) reducing agent, in this reaction.
ZnO + C → Zn + CO (i) An oxidizing agent is a reactant that gains electrons and undergoes reduction during a redox reaction. In the reaction shown above, the oxidizing agent is zinc oxide as it...
What is a decomposition reaction? Give an example of a decomposition reaction. Describe an activity to illustrate such a reaction by heating.
A decomposition reaction occurs when a substance is broken down or split into two or more components. They are divided into three categories. Thermal decomposition, electrolytic decomposition, and...
(a) What is the colour of ferrous sulphate crystals? How does this colour change after heating?
(b) Name the product formed on strongly heating ferrous sulphate crystals. What type of chemical reaction occurs in this change?
(a) Ferrous sulphate crystals are green in colour but their colour changes to white when heated. This is because the water in the crystals evaporates and anhydrous ferrous sulphate is produced,...
(a) Give an example of an endothermic reaction?
(b) Is oxidation an exothermic or an endothermic reaction?
(c) Explain, by giving an example, how oxidation and reduction processed side by side.
(a) An endothermic process is one in which heat is absorbed during the reaction. For example, burning carbon in the presence of oxygen produces carbon dioxide and releases a significant quantity of...
(a) What is a redox reaction? Explain with an example. (b) When a magnesium ribbon burns in air with a dazzling flame and forms a white ash, is magnesium oxidised or reduced? Why? (c) In the reaction represented by the equation: MnO2 + 4HCl → MnCl2 +2H2O +Cl2
(i) Name the substance oxidized
(ii) Name the oxidizing agent
(iii) Name the substance reduced
(iv) Name the reducing agent
A redox reaction is a reaction when both oxidation and reduction take place simultaneously. An example for a redox reaction is as follows:- SO3(aq) + MnO4(aq) → SO4(aq) + Mn Here, Sulphur trioxide...
What is an oxidation reaction? Identify in the following reaction (i) the substance oxidized, and (ii) substance reduced: ZnO + C → Zn +CO
Oxidation is the process of losing an electron by an atom. Alternatively, oxygen can be added to a substance to raise the fraction of oxygen in its molecule. Oxidation can even be accomplished...
When current is switched on and switched off in a coil, a current is induced in another coil kept near it. What is this phenomenon known as?
Answer: Electromagnetic induction is the term used to describe the phenomena in which current is switched on and off in a coil in order to induce a current in another coil.
When a wire is moved up and down in a magnetic field, a current is induced in the wire. What is this phenomenon known as?
Answer: Electromagnetic induction is the term used to describe the phenomena in which a wire moves up and down in a magnetic field in order to generate a current in the wire.
What is the function of brushes in an electric generator?
Answer: Among their many functions, the brushes of an electric generator are responsible for transferring electricity from the coil to the load.
State, whether the following statements are true or false:
a) A generator works on the principle of electromagnetic induction.
b) A motor works on the principle of electromagnetic induction.
Answer: a) True Explanation: A generator produces electricity by rotating a coil in a magnetic field. An electric generator (dynamo) turns mechanical energy into electrical energy. Generators do not...
What change should be made in an AC generator so that it may become a DC generator?
Answer: To convert an alternating current generator into a direct current generator, the slip rings must be replaced with a commutator.
What type of generator is used at power stations?
Answer: At power plants, alternating current generators are used.
What condition is necessary for the production of current by electromagnetic induction?
Answer: The movement of the wire in relation to the magnet is a necessary condition for the generation of current through electromagnetic induction to occur.
Name the rule which gives the direction of induced current.
Answer: Fleming's right-hand rule is employed in order to determine the direction of the induced voltage.
Name the phenomenon which is made use of in an electric generator.
Answer: Electromagnetic induction is a phenomenon that is used in the production of electricity by electric generators.
Out of an AC generator and a DC generator:
a) which one uses a commutator (split rings)?
b) which one uses slip rings?
Answer: a) A commutator is used by a direct current generator. b) Slip rings are used by the alternating current generator (AC generator).
Name the device which converts mechanical energy into electric energy.
Answer: Electric generators are devices that are used to transform mechanical energy into electrical energy. They are also known as power generators.
Fill in the following blanks with suitable words:
(a) The addition of oxygen to a substance is called ______ whereas removal of oxygen is called ______.
(b) The addition of hydrogen to a substance is called ______ whereas removal of hydrogen is called ______.
(c) Anti-oxidants are often added to fat containing foods to prevent ______ due to oxidation
Answer: (a) Oxidation; reduction. (b) Reduction; oxidation. (c) Rancidity.
When SO2 gas is passed through saturated solution of H2S, the following reaction occurs: ![Rendered by QuickLaTeX.com \[\mathbf{S}{{\mathbf{O}}_{\mathbf{2}}}~+\text{ }\mathbf{2}{{\mathbf{H}}_{\mathbf{2}}}\mathbf{S}\text{ }\to \text{ }\mathbf{2}{{\mathbf{H}}_{\mathbf{2}}}\mathbf{O}\text{ }+\mathbf{3S}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-f253585babb374c2b6e61729cf8a446f_l3.png)
In this reaction, which substance is oxidized and which one is reduced?
SO2 + 2H2S → 2H2O +3S In the reaction above, H2S got oxidized as Hydrogen was removed from the molecule while SO2 got reduced because Oxygen was removed.
Identify the component oxidized in the following reaction: ![Rendered by QuickLaTeX.com \[{{\mathbf{H}}_{\mathbf{2}}}\mathbf{S}\text{ }+\mathbf{C}{{\mathbf{l}}_{\mathbf{2}}}\to \text{ }\mathbf{S}\text{ }+\mathbf{2HCl}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-5a825f6d792d8b4bee77b84ccd034b34_l3.png)
H2S is oxidized in the above reaction as Hydrogen is being removed from H2S to release sulphur.
In the following reaction between lead sulphide and hydrogen peroxide: ![Rendered by QuickLaTeX.com \[\mathbf{PbS}\left( \mathbf{s} \right)\text{ }+\text{ }\mathbf{4}{{\mathbf{H}}_{\mathbf{2}}}{{\mathbf{O}}_{\mathbf{2}}}\left( \mathbf{aq} \right)\text{ }\to \text{ }\mathbf{PbS}{{\mathbf{O}}_{\mathbf{4}}}\left( \mathbf{s} \right)\text{ }+\mathbf{4}{{\mathbf{H}}_{\mathbf{2}}}\mathbf{O}\left( \mathbf{l} \right)\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-9da3bbdbcec1b52f5114c4f9aade8a63_l3.png)
(a) Which substance is reduced? (b) Which substance is oxidized?
(a) H2O2 is reduced. (b) PbS is oxidised.
What type of reactions are represented by the following equations?
![Rendered by QuickLaTeX.com \[\left( \mathbf{a} \right)\text{ }\mathbf{CaO}\text{ }+\text{ }\mathbf{C}{{\mathbf{O}}_{\mathbf{2}}}\to \text{ }\mathbf{CaC}{{\mathbf{O}}_{\mathbf{3}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-0a64bd62fb54bbd0edf1b63b6683ee24_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{b} \right)\text{ }\mathbf{2Na}\text{ }+\mathbf{2}{{\mathbf{H}}_{\mathbf{2}}}\mathbf{O}\text{ }\to \text{ }\mathbf{2NaOH}\text{ }+{{\mathbf{H}}_{\mathbf{2}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-ca02eab857f32a5212e1bec9e28dde03_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{c} \right)\text{ }\mathbf{Mg}\text{ }+\text{ }\mathbf{CuS}{{\mathbf{O}}_{\mathbf{4}}}\to \text{ }\mathbf{MgS}{{\mathbf{O}}_{\mathbf{4}}}~+\text{ }\mathbf{Cu}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-1fc4d1026bbc1af1b8dabbefee1e3766_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{d} \right)\text{ }\mathbf{N}{{\mathbf{H}}_{\mathbf{4}}}\mathbf{N}{{\mathbf{O}}_{\mathbf{2}}}\to \text{ }{{\mathbf{N}}_{\mathbf{2}}}~+\mathbf{2}{{\mathbf{H}}_{\mathbf{2}}}\mathbf{O}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-ec63045a06b69bdeacca721fae9d3a06_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{e} \right)\text{ }\mathbf{CuS}{{\mathbf{O}}_{\mathbf{4}}}~+\text{ }\mathbf{2NaOH}\text{ }\to \mathbf{Cu}{{\left( \mathbf{OH} \right)}_{\mathbf{2}}}~+\mathbf{N}{{\mathbf{a}}_{\mathbf{2}}}\mathbf{S}{{\mathbf{O}}_{\mathbf{4}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-66adb56ae15b498d389cdc9659a64a2f_l3.png)
(a) Combination reaction, since calcium oxide combines with carbon dioxide to calcium carbonate. (b) Displacement reaction, since sodium replaces oxygen to give sodium hydroxide. (c) Displacement...
Which of the following is a combination and which is a displacement reaction.
![Rendered by QuickLaTeX.com \[\left( \mathbf{a} \right)\text{ }\mathbf{C}{{\mathbf{l}}_{\mathbf{2}~}}+\mathbf{2KI}\text{ }\to \text{ }\mathbf{2KCl}\text{ }+{{\mathbf{I}}_{\mathbf{2}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-4dc4be4dcdc2a604694c564896fcc2aa_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{b} \right)\text{ }\mathbf{2K}\text{ }+\text{ }\mathbf{C}{{\mathbf{l}}_{\mathbf{2}~}}\to \text{ }\mathbf{2KCl}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-7c0994c2a89c00e7940167bc34f57534_l3.png)
(a) Displacement reaction, since Chlorine replaces Iodine to combine with POtassium to give Potassium chloride. (b) Combination reaction since Potassium and chloride combine to form two molecules of...
Balance the following chemical equations:
![Rendered by QuickLaTeX.com \[\left( \mathbf{a} \right)\text{ }\mathbf{FeS}{{\mathbf{O}}_{\mathbf{4}}}\to \text{ }\mathbf{F}{{\mathbf{e}}_{\mathbf{2}}}{{\mathbf{O}}_{\mathbf{3}}}~+\text{ }\mathbf{S}{{\mathbf{O}}_{\mathbf{2}}}~+\text{ }\mathbf{S}{{\mathbf{O}}_{\mathbf{3}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-39a391e2efc0e61cf16f7278f41bd043_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{b} \right)\text{ }\mathbf{Pb}{{\left( \mathbf{N}{{\mathbf{O}}_{\mathbf{3}}} \right)}_{\mathbf{2}}}\left( \mathbf{s} \right)\text{ }\to \mathbf{PbO}\left( \mathbf{s} \right)\text{ }+\text{ }\mathbf{N}{{\mathbf{O}}_{\mathbf{2}}}\left( \mathbf{g} \right)\text{ }+\text{ }{{\mathbf{O}}_{\mathbf{2}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-d80c527fb417f074f02fec6a6299e69f_l3.png)
The given chemical equations have been balanced as follows: (a) 2FeSO4→ Fe2O3 + SO2 + SO3 In this reaction, Ferrous sulphate decomposes to give Ferric oxide. Sulphur dioxide and sulphur trioxide....
What type of chemical reactions is represented by the following equations?
(i) A + BC → AC+ B
(ii) A +B → C
(iii) X → Y+ Z
(iv) PQ + RS → PS + RQ
(v) A2O3 + 2B → B2O3 + 2A
(i) Displacement reaction, since A replaces B to combine to C giving AC. (ii) Combination reaction since A and B combine to give a product C which is different from both the reactants. (iii)...
What type of chemical reaction stake place when:
(a) A magnesium wire is burnt in air?
(b) Lime-stone is heated?
(c) Silver bromide is exposed to sunlight?
(d) Electricity is passed through water?
(e) Ammonia and hydrogen chloride are mixed?
(a) Combination reaction, since magnesium reacts with oxygen in this reaction. (b) Decomposition reaction, since on heating limestone decomposes to give calcium oxide and carbon dioxide. (c)...
What type of reactions are represented by the following equations?
![Rendered by QuickLaTeX.com \[\left( \mathbf{i} \right)\text{ }\mathbf{CaC}{{\mathbf{O}}_{\mathbf{3}}}\to ~\text{ }\mathbf{CaO}\text{ }+\text{ }\mathbf{C}{{\mathbf{O}}_{\mathbf{2}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-0c92930afac1ec5967f99a1ab56a8bb7_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{ii} \right)\text{ }\mathbf{CaO}\text{ }+\text{ }{{\mathbf{H}}_{\mathbf{2}}}\mathbf{O}\text{ }\to ~\text{ }\mathbf{Ca}{{\left( \mathbf{OH} \right)}_{\mathbf{2}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-09a5e04c0d57cc97c3ed951a5fda53fb_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{iii} \right)\text{ }\mathbf{2FeS}{{\mathbf{O}}_{\mathbf{4}}}\to ~\mathbf{F}{{\mathbf{e}}_{\mathbf{2}}}{{\mathbf{O}}_{\mathbf{3}}}~+\text{ }\mathbf{S}{{\mathbf{O}}_{\mathbf{2}}}+\text{ }\mathbf{S}{{\mathbf{O}}_{\mathbf{3}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-8017c8e89d01e684bcf61c0f1de70693_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{iv} \right)\text{ }\mathbf{N}{{\mathbf{H}}_{\mathbf{4}}}\mathbf{Cl}\text{ }\to ~\text{ }\mathbf{N}{{\mathbf{H}}_{\mathbf{3}}}~+\text{ }\mathbf{HCl}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-dfa6e7a26dfa378a6a20c25533177fab_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{v} \right)\text{ }\mathbf{2Ca}\text{ }+\text{ }{{\mathbf{O}}_{\mathbf{2}}}\to ~\text{ }\mathbf{2CaO}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-4190dac4b3d24fa80d042d143aeff889_l3.png)
Answer: (i) Decomposition reaction, since calcium carbonate decomposes to gove calcium oxide and carbon dioxide in the reaction. (ii) Combination reaction, since Calcium oxide combines with water to...
Answer the following questions:
(a) If the earth did not have an atmosphere, would its average surface temperature be higher or lower than what it is now?
(b) Some scientists have predicted that a global nuclear war on the earth would be followed by a severe ‘nuclear winter’ with a devastating effect on life on earth. What might be the basis of this prediction?
(a) There would be no greenhouse effect if there was no atmosphere. As a result, the earth's temperature would plummet. (b) Smoke clouds from a worldwide nuclear war might potentially cover large...
Answer the following questions:
(a) Optical and radio telescopes are built on the ground but X-ray astronomy is possible only from satellites orbiting the earth. Why?
(b) The small ozone layer on top of the stratosphere is crucial for human survival. Why?
(a) X-rays are absorbed by the atmosphere, although visible and radio waves can get through. (b) The ozone layer absorbs ultraviolet energy from the sun, preventing it from reaching the earth's...
Answer the following questions:
(a) Long-distance radio broadcasts use short-wave bands. Why?
(b) It is necessary to use satellites for long-distance TV transmission. Why?
(a) Ionosphere reflects waves in the shortwave bands. (b) The frequency and energy of television transmissions are both high. As a result, the ionosphere does not fully reflect it. The TV...
Given below are some famous numbers associated with electromagnetic radiations in different contexts in physics. State the part of the electromagnetic spectrum to which each belongs. 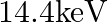 [energy of a particular transition in
[energy of a particular transition in 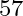 Fe nucleus associated with a famous high resolution spectroscopic method (Mössbauer spectroscopy)].
Fe nucleus associated with a famous high resolution spectroscopic method (Mössbauer spectroscopy)].
X-rays region
Given below are some famous numbers associated with electromagnetic radiations in different contexts in physics. State the part of the electromagnetic spectrum to which each belongs.
(a) 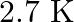 [temperature associated with the isotropic radiation filling all space-thought to be a relic of the ‘big-bang’ origin of the universe].
[temperature associated with the isotropic radiation filling all space-thought to be a relic of the ‘big-bang’ origin of the universe].
(b) 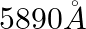 –
– 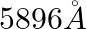 [double lines of sodium]
[double lines of sodium]
(a) Microwave (b) Visible light
Given below are some famous numbers associated with electromagnetic radiations in different contexts in physics. State the part of the electromagnetic spectrum to which each belongs.
(a) 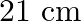 (wavelength emitted by atomic hydrogen in interstellar space).
(wavelength emitted by atomic hydrogen in interstellar space).
(b) 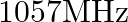 (frequency of radiation arising from two close energy levels in hydrogen; known as Lamb shift).
(frequency of radiation arising from two close energy levels in hydrogen; known as Lamb shift).
(a) Radio waves (b) Radio waves
Use the formula 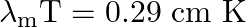 to obtain the characteristic temperature ranges for different parts of the electromagnetic spectrum. What do the numbers that you obtain tell you?
to obtain the characteristic temperature ranges for different parts of the electromagnetic spectrum. What do the numbers that you obtain tell you?
The given equation is, $\lambda_{\mathrm{m}} \mathrm{T}=0.29 \mathrm{~cm} \mathrm{~K}$ $\Rightarrow \mathrm{T}=\left(0.29 / \lambda_{\mathrm{m}}\right) \mathrm{cm} \mathrm{K}$ where, $T$ is the...
About 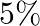 of the power of a
of the power of a 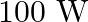 light bulb is converted to visible radiation. What is the average intensity of visible radiation.
light bulb is converted to visible radiation. What is the average intensity of visible radiation.
(a) at a distance of 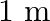 from the bulb?
from the bulb?
(b) at a distance of 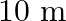 ? Assume that the radiation is emitted isotropically and neglect reflection.
? Assume that the radiation is emitted isotropically and neglect reflection.
(a) Average intensity of the visible radiation is given by the expression, $I=P^{\prime} / 4 \pi d^{2}$ So, the power of the visible radiation will be $P^{\prime}=(5 / 100) \times 100=5 \mathrm{~W}$...
Suppose that the electric field part of an electromagnetic wave in vacuum is 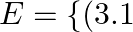
![Rendered by QuickLaTeX.com \left.\mathrm{N} / \mathrm{C}) \cos \left[(1.8 \mathrm{rad} / \mathrm{m}) \mathrm{y}+\left(5.4 \times 10^{6} \mathrm{rad} / \mathrm{s}\right) \mathrm{t}\right]\right\}^{\wedge} \mathrm{i}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-aa0dc5ee496338ec62b17ccfc2c0969c_l3.png) . Write an expression for the magnetic field part of the wave.
. Write an expression for the magnetic field part of the wave.
Magnetic wave is directed along the negative z-direction. Thus, B = Bocos(ky+ωt)k $B_{z}=B_{0} \cos (k y+\omega t)^{2} k=\left{(10.3 \mathrm{nT}) \cos \left[(1.8 \mathrm{rad} / \mathrm{m})...
Suppose that the electric field part of an electromagnetic wave in vacuum is 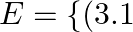
![Rendered by QuickLaTeX.com \left.\mathrm{N} / \mathrm{C}) \cos \left[(1.8 \mathrm{rad} / \mathrm{m}) \mathrm{y}+\left(5.4 \times 10^{6} \mathrm{rad} / \mathrm{s}\right) \mathrm{t}\right]\right\}^{\wedge} \mathrm{i}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-aa0dc5ee496338ec62b17ccfc2c0969c_l3.png)
(a) What is the frequency v?
(b) What is the amplitude of the magnetic field part of the wave?
(a) Frequency is calculated as $v=\omega / 2 \pi=5.4 \times 10^{6} /(2 \times 3.14)=0.859 \times 10^{6} \mathrm{~Hz}$ (b) Amplitude of the magnetic field can be calculated...
Suppose that the electric field part of an electromagnetic wave in vacuum is 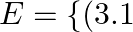
![Rendered by QuickLaTeX.com \left.\mathrm{N} / \mathrm{C}) \cos \left[(1.8 \mathrm{rad} / \mathrm{m}) \mathrm{y}+\left(5.4 \times 10^{6} \mathrm{rad} / \mathrm{s}\right) \mathrm{t}\right]\right\}^{\wedge} \mathrm{i}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-aa0dc5ee496338ec62b17ccfc2c0969c_l3.png)
(a) What is the direction of propagation?
(b) What is the wavelength  ?
?
(a) The motion is going in the opposite direction of the y-axis. To put it another way, along -j. (b) The given equation is compared with the equation and we get, $E=E_{0} \cos (k y+\omega t)$...
In a plane electromagnetic wave, the electric field oscillates sinusoidally at a frequency of 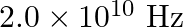 and amplitude
and amplitude 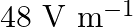 . Show that the average energy density of the
. Show that the average energy density of the  field equals the average energy density of the B field.
field equals the average energy density of the B field. ![Rendered by QuickLaTeX.com \left[\mathrm{c}=3 \times 10^{8} \mathrm{~m} \mathrm{~s}^{-1}\right]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-96ac54c20f4c7b80bd9bf00b657be717_l3.png)
Frequency of the electromagnetic wave is given as $\mathrm{v}=2 \times 10^{10} \mathrm{~Hz}$ Electric field amplitude is given as $E_{0}=48 \vee \mathrm{m}^{-1}$ Speed of light is known as $c=3...
In a plane electromagnetic wave, the electric field oscillates sinusoidally at a frequency of 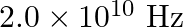 and amplitude
and amplitude 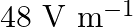 .
.
(a) What is the wavelength of the wave?
(b) What is the amplitude of the oscillating magnetic field?
Frequency of the electromagnetic wave is given as $\mathrm{v}=2 \times 10^{10} \mathrm{~Hz}$ Electric field amplitude is given as $E_{0}=48 \vee \mathrm{m}^{-1}$ Speed of light is known as $c=3...
In the refining of silver, the recovery of silver from silver nitrate solution involved displacement by copper metal. Write down the chemical equation of the reaction involved.
Chemical equation for the recovery of silver from silver nitrate solution which involves displacement by copper metal is as follows:- 2AgNO3 (aq) + Cu (s) → Cu(NO3)2 (aq) + 2Ag (s)
The terminology of different parts of the electromagnetic spectrum is given in the text. Use the formula 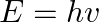 (for the energy of a quantum of radiation: photon) and obtain the photon energy in units of eV for different parts of the electromagnetic spectrum. In what way are the different scales of photon energies that you obtain related to the sources of electromagnetic radiation?
(for the energy of a quantum of radiation: photon) and obtain the photon energy in units of eV for different parts of the electromagnetic spectrum. In what way are the different scales of photon energies that you obtain related to the sources of electromagnetic radiation?
The energy of a photon is represented by the expression, $\mathrm{E}=\mathrm{hv}=\frac{h c}{\lambda}$ Where, $\mathrm{h}$ is the planck's constant with a value of$6.6 \times 10^{-34} J_{S}$...
Write one equation each for the decomposition reactions where energy is supplied in the form of (a) heat, (b) light, and (c) electricity.
(a) When heat is applied, a decomposition reaction occurs. 2Pb(NO3)2→ 2PbO + 4NO2 + O2 (b) Decomposition reaction in the presence of light 2AgCl → 2Ag + Cl2 (c) Decomposition reaction with the...
Suppose that the electric field amplitude of an electromagnetic wave is 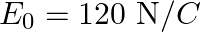 and that its frequency is
and that its frequency is 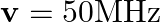 .(a) Determine
.(a) Determine 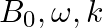 and
and  (b) Find expressions for
(b) Find expressions for  and
and  .
.
Electric field amplitude is given as $E_{0}=120 \mathrm{~N} / C$ Frequency of source is given as $v=50 \mathrm{MHz}=50 \times 10^{6} \mathrm{~Hz}$ Speed of light is known as $c=3 \times 10^{8}...
Name two anti-oxidants which are usually added to fat and oil containing foods to prevent rancidity.
Antioxidants are utilized as preservatives in fat-containing foods to delay oxidation-induced rancidity. Antioxidants with longer half-lives, such as butylated hydroxytoluene (BHT) and butylated...
The amplitude of the magnetic field part of a harmonic electromagnetic wave in vacuum is  . What is the amplitude of the electric field part of the wave?
. What is the amplitude of the electric field part of the wave?
Amplitude of magnetic field of an electromagnetic wave in a vacuumis given to us as, $B_{0}=510 n T=510 \times 10^{-9} T$ Speed of light in vacuum is known as $\mathrm{c}=3 \times 10^{8} \mathrm{~m}...
What type of chemical reaction is used to extract metals from their natural compounds like oxides or chlorides?
A decomposition reaction is used to extract several metals from their naturally occurring compounds such as oxide and chloride. Example: 2NaCl → 2Na + Cl2
A charged particle oscillates about its mean equilibrium position with a frequency of 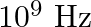 . What is the frequency of the electromagnetic waves produced by the oscillator?
. What is the frequency of the electromagnetic waves produced by the oscillator?
The frequency of an electromagnetic wave generated by the oscillator is the same as that of a charged particle oscillating around its mean position, which is $10^{9} \mathrm{~Hz}$.
Give one example of a decomposition reaction that is carried out: (a) With electricity (b) By applying heat
(a) Electricity decomposes into hydrogen and oxygen gas when it passes through acidified water. 2H2 O → 2H2 + O2 (a) Thermal decomposition occurs when heat is applied. An example of thermal...
A radio can tune in to any station in the  to
to  bands. What is the corresponding wavelength band?
bands. What is the corresponding wavelength band?
The minimum frequency for a radio to tune is given as $v_{1}=7.5 M H z=7.5 \times 10^{6} H z$ $=\frac{3 \times 10^{3}}{7.5 \times 10^{8}}=40 \mathrm{~m}$ $\lambda_{2}=\frac{c}{v_{2}}$ $=\frac{3...
A plane electromagnetic wave travels in vacuum along the z-direction. What can you say about the directions of its electric and magnetic field vectors? If the frequency of the wave is 30 MHz, what is its wavelength?
In a vacuum, the electromagnetic wave travels in the z-direction. In the $x-y$ plane, there is an electric field (E) and a magnetic field $(\mathrm{H})$. They are perpendicular to one other....
Explain why food products containing fats and oils (like potato chips) are packaged in nitrogen.
Nitrogen is used to flush the packet of chips or foods containing fats and oils. This is because nitrogen acts as an antioxidant. The food is packaged to keep it from getting oxidizing. When we pack...
What physical quantity is the same for  -rays of wavelength
-rays of wavelength 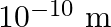 , the red light of wavelength
, the red light of wavelength  and radiowaves of wavelength
and radiowaves of wavelength 
All wavelengths of light travel at the same speed in a vacuum. In the vacuum, it is unaffected by the wavelength.





